|
|
| Figure 1. A representative axial section of a segmented training image. | Figure 2. Two representative axial sections showing the results of manual segmentation of white-matter lesions (a, c) and the method described in this section (b, d). |
|
|
| Figure 1. A representative axial section of a segmented training image. | Figure 2. Two representative axial sections showing the results of manual segmentation of white-matter lesions (a, c) and the method described in this section (b, d). |
|
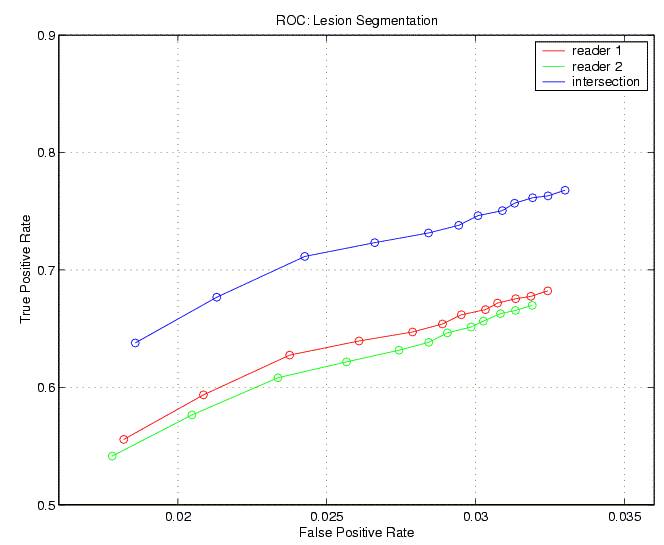
| Figure 3. ROC curves for the white-matter-lesion segmentation algorithm, compared to two neuroradiologists and their intersection (regions of agreement). |